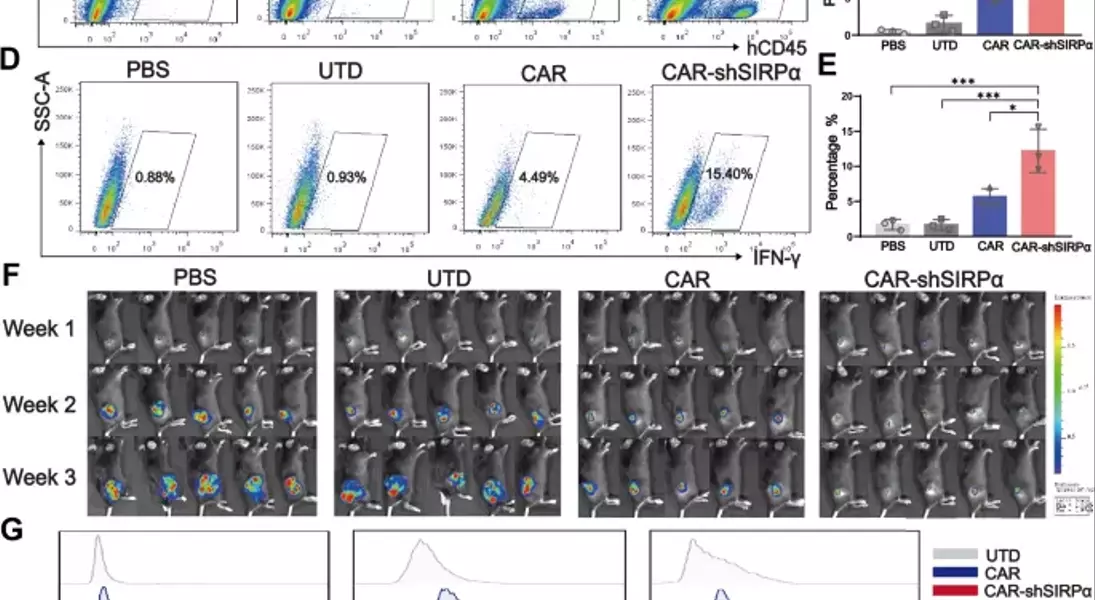
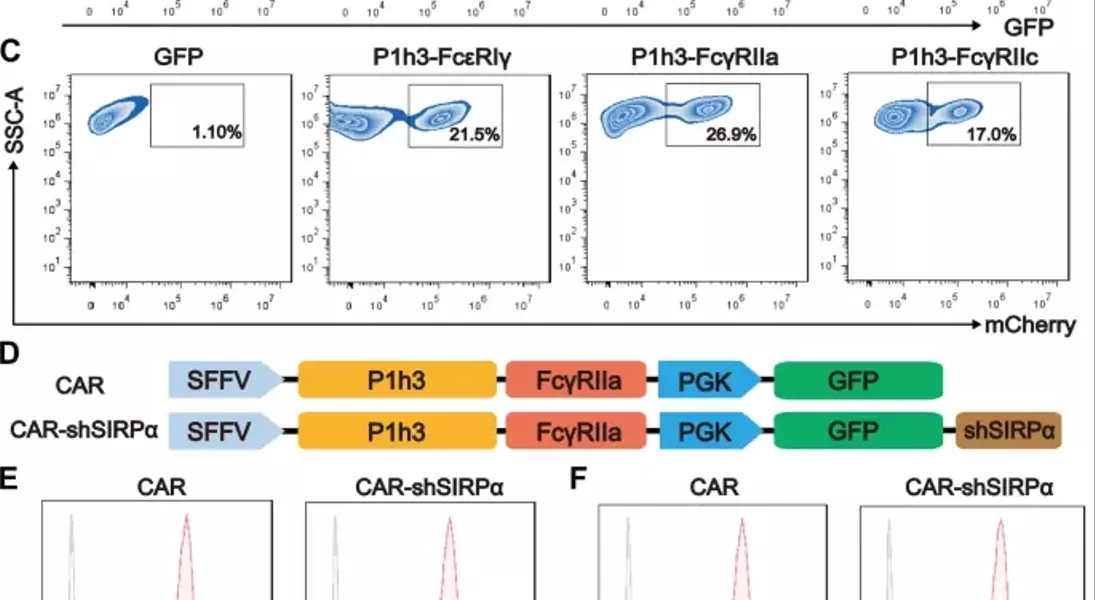
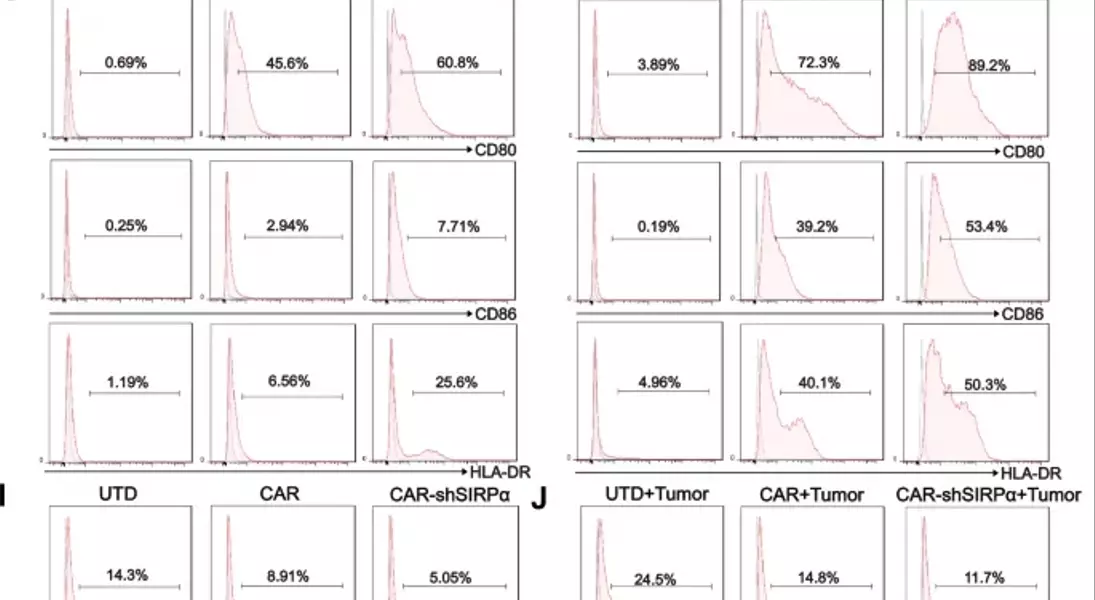
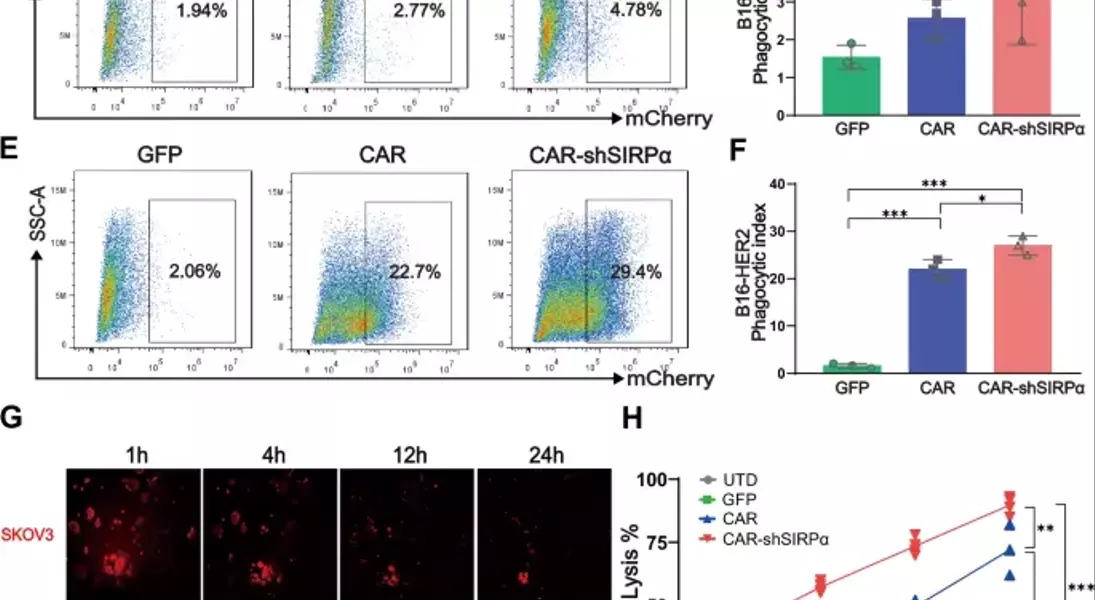
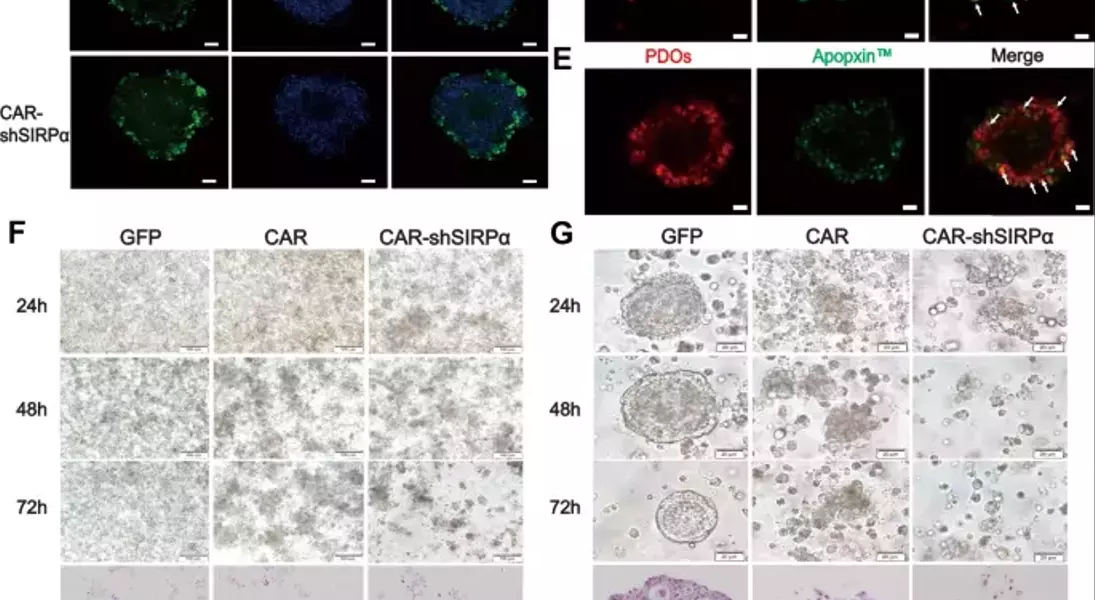
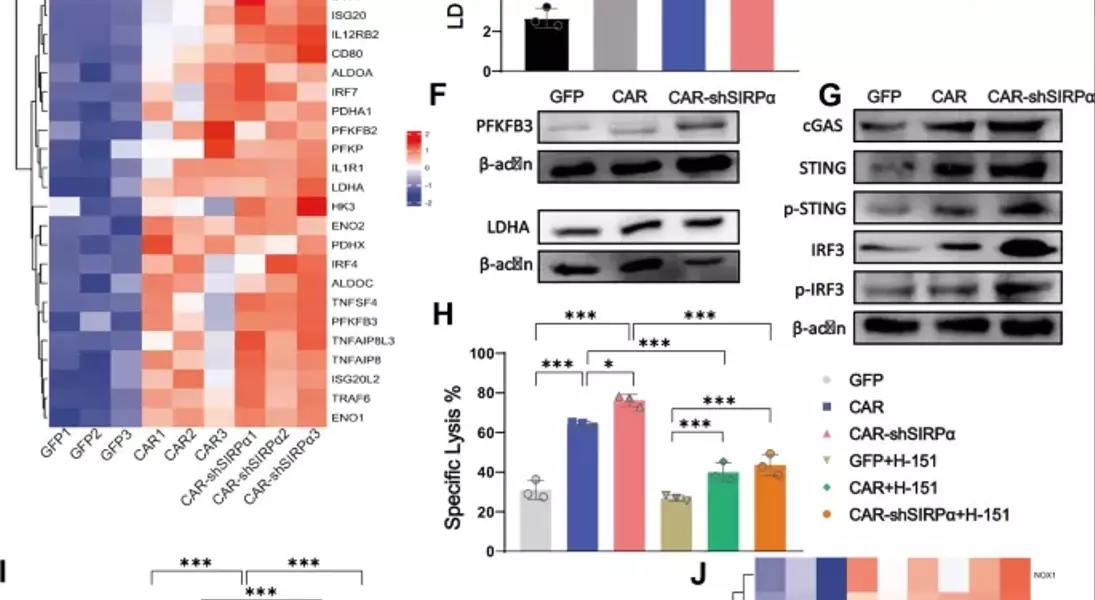



Supercharged CAR-M Cells: Unlocking the Potential of Macrophage-Mediated ImmunotherapyAdoptive cell therapy, particularly chimeric antigen receptor T-cell (CAR-T) therapy, has made remarkable progress in the treatment of hematological malignancies. However, its efficacy against solid tumors has not met expectations, primarily because of challenges related to tumor infiltration and the suppressive nature of the immune microenvironment. Macrophages, on the other hand, exhibit a strong capacity for tumor infiltration and constitute the predominant immune cell population in most solid tumors. This makes them ideal vectors for CAR constructs, and recent research has garnered increasing attention in the field of cancer immunotherapy.## Unleashing the Power of Macrophages in Cancer Immunotherapy### Harnessing the Phagocytic Prowess of CAR-M CellsPhagocytosis is a vital mechanism by which macrophages eliminate tumor cells and activate the immune system. This process is regulated by receptor-ligand interactions, which signal that macrophages either promote or inhibit phagocytosis. The CD47-SIRPα signaling pathway serves as a key phagocytic checkpoint, with tumor cells often overexpressing CD47 to evade phagocytosis. By incorporating the Fc receptor FcγRIIa into the CAR structure, researchers have been able to enhance the targeted phagocytic activity of macrophages, enabling them to effectively engulf and destroy tumor cells.### Silencing SIRPα: A Novel Strategy to Boost CAR-M PotencyWhile the incorporation of FcγRIIa provides an "eat-me" signal, tumor cells employ additional layers of CD47-SIRPα "don't-eat-me" signals to deceive and evade phagocytosis by CAR-M cells. To counteract this, researchers have introduced a specific short hairpin RNA (shRNA) to silence SIRPα, disrupting the CD47-SIRPα signaling axis. This strategic combination of the "eat-me" signal from FcγRIIa and the suppression of the "don't-eat-me" signal via SIRPα inhibition has endowed CAR-shSIRPα-M cells with increased phagocytic activity and an M1-like phenotype, resulting in significant cytotoxic effects on HER2-positive tumor cells.### Unleashing Multifaceted Antitumor MechanismsThe enhanced phagocytosis of tumor cells by CAR-shSIRPα-M cells activates innate immune response pathways, leading to the activation of inflammatory signaling cascades, such as the NF-κB and cGAS-STING pathways. This, in turn, triggers the production of proinflammatory cytokines, reactive oxygen species (ROS), and nitric oxide (NO), further amplifying the antitumor effects of these modified macrophages.### Fostering a Robust Adaptive Immune ResponseIn addition to their direct tumor-killing capabilities, CAR-shSIRPα-M cells have also been shown to enhance cytotoxic T-cell infiltration into the tumor microenvironment, thereby stimulating a more durable adaptive immune response. By disrupting the CD47-SIRPα signaling axis, these modified macrophages not only eliminate tumor cells through phagocytosis but also facilitate the activation and recruitment of cytotoxic T cells, creating a synergistic antitumor effect.### Impressive In Vivo Efficacy and Safety ProfileThe potent antitumor activity of CAR-shSIRPα-M cells has been demonstrated in various in vivo models, including subcutaneous, intraperitoneal, and lung metastasis tumor models. These modified macrophages significantly inhibited tumor growth, prolonged the survival of tumor-bearing mice, and exhibited a favorable safety profile, with no apparent toxicity observed in vital organs.## Unlocking the Full Potential of CAR-M Cells in Solid Tumor ImmunotherapyThe strategic engineering of CAR-M cells, particularly through the silencing of SIRPα, has emerged as a promising approach to overcome the challenges associated with solid tumor immunotherapy. By harnessing the multifaceted antitumor mechanisms of these modified macrophages, including enhanced phagocytosis, activation of innate immune pathways, and promotion of adaptive immune responses, researchers have demonstrated the potential to achieve durable and effective cancer treatment. As the field of CAR-M cell therapy continues to evolve, further advancements in this innovative approach may pave the way for transformative breakthroughs in the management of solid tumors.
