


Across the United States, elderly patients are increasingly using costly bandages made from dried placental tissue. These thin patches, designed to treat persistent wounds, come with an exorbitant price tag that can exceed thousands of dollars per square inch. While some studies suggest these "skin substitutes" promote healing for certain injuries, the market has been flooded in recent years with numerous unstudied and expensive products. Exploiting a loophole in Medicare regulations, companies producing these bandages set inflated prices, while doctors often buy them at discounted rates but charge Medicare the full amount, pocketing the difference. This financial incentive has led to widespread misuse, with many patients receiving unnecessary treatments. Experts claim this represents one of the most significant examples of Medicare waste in history, with spending on skin substitutes surpassing $10 billion in 2024.
Rising Costs of Skin Substitutes Alarm Healthcare Experts
In the heart of a rapidly evolving healthcare landscape, concerns have arisen over the escalating costs associated with specialized wound care products. During the past decade, the use of placenta-based bandages has surged dramatically, particularly among senior citizens covered by Medicare. These advanced skin substitutes, crafted from meticulously processed placental materials, promise enhanced recovery for challenging wounds. However, their astronomical pricing—averaging nearly $6,000 per square inch—has raised eyebrows across the medical community.
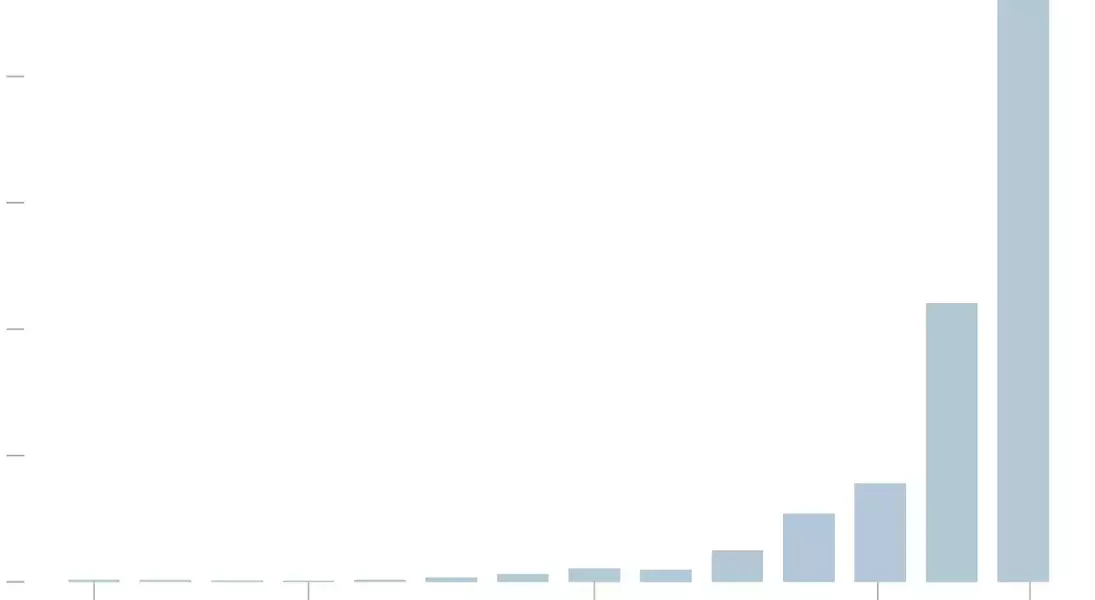
Since 2011, annual expenditures on such products have skyrocketed, reaching a staggering $10 billion by 2024. This dramatic increase is partly attributed to regulatory gaps within Medicare's framework, allowing manufacturers to continually raise prices without justification. Simultaneously, some physicians exploit these discrepancies by acquiring the bandages at reduced rates yet billing Medicare for the full cost, leading to substantial profit margins.
The consequences of this practice extend beyond financial implications. Many seniors, unaware of alternative treatment options or the true necessity of these products, undergo unnecessary treatments, exacerbating the issue of resource misallocation. With private insurers largely refusing coverage due to insufficient evidence of efficacy, Medicare remains virtually the sole payer, further amplifying the problem.
From a broader perspective, experts warn that this trend not only jeopardizes the sustainability of Medicare funding but also raises ethical questions regarding equitable access to healthcare resources.
As discussions continue, stakeholders emphasize the urgent need for stricter oversight and more transparent pricing structures to ensure responsible utilization of taxpayer-funded programs.
This situation exemplifies how regulatory loopholes can lead to massive inefficiencies in public healthcare systems. It underscores the importance of revisiting existing policies to better align them with current medical advancements and economic realities.
Reflections on the Ethical Dilemma Surrounding Medical Pricing
From a journalistic standpoint, this case highlights a pressing ethical dilemma within the healthcare industry. The unchecked escalation of prices for specialized medical products, coupled with exploitative practices by both manufacturers and practitioners, poses serious challenges to the integrity of our healthcare system. It forces us to reconsider whether current regulatory frameworks are adequately equipped to handle rapid technological innovations in medicine.
For readers, it serves as a poignant reminder of the complexities involved in balancing innovation with affordability in healthcare delivery. It prompts deeper reflection on how society values health and well-being versus profit motives, encouraging all parties—patients, providers, policymakers—to advocate for reforms fostering greater transparency and accountability.
